About ADS-Limathon Limited
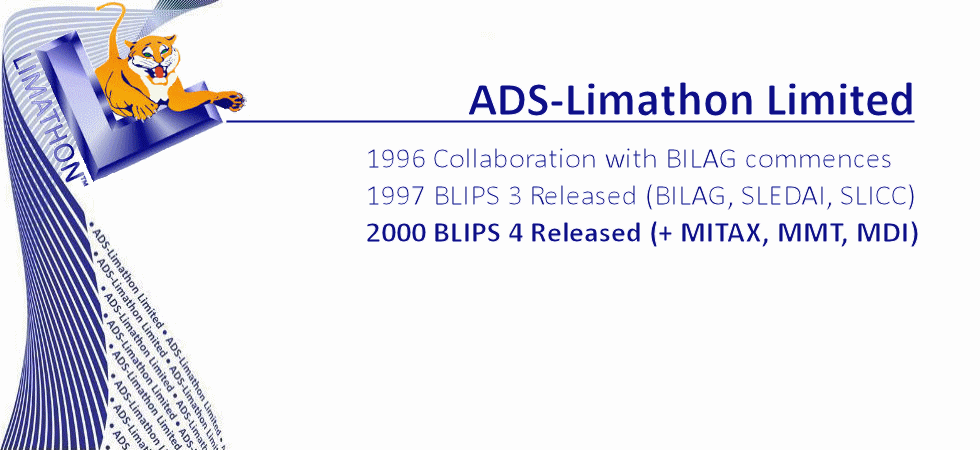
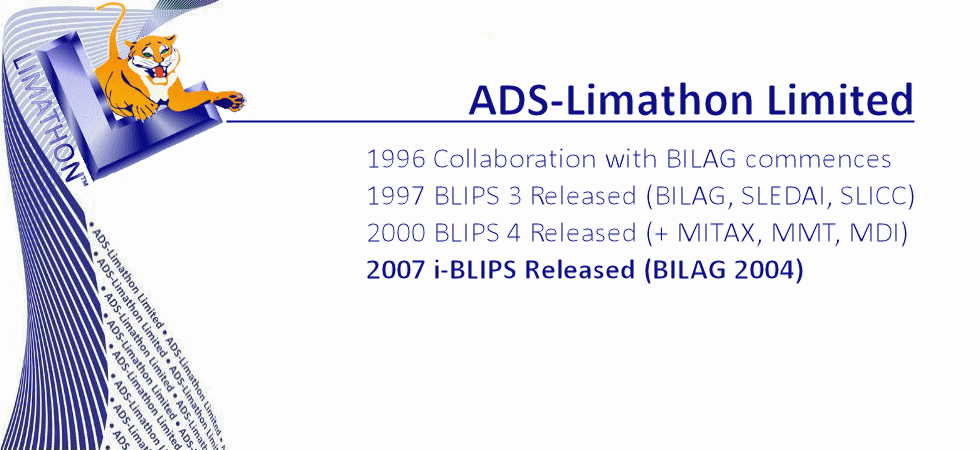
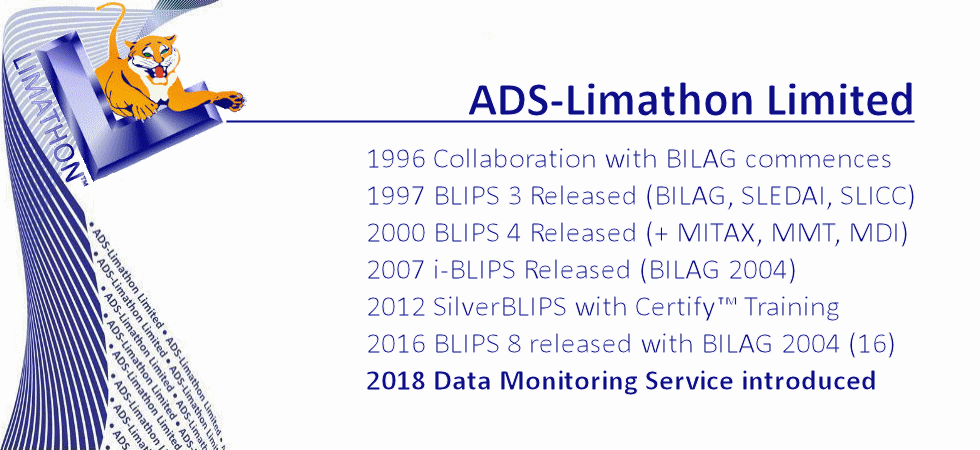
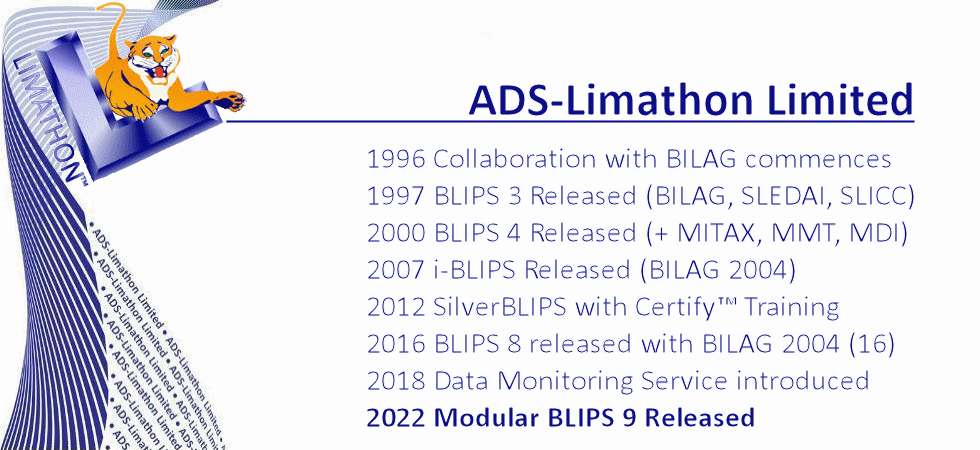
Analytical Database Systems Limited was founded in 1994, in Sheffield UK, by Joyce and Gordon Hamilton. A year later a second company Limathon Limited was formed, to develop laboratory intrumentation software, and in 1999 both companies amalgamated to form ADS-Limathon Limited. ADS began collaborating with the British Isles Lupus Assessment Group (BILAG) in 1996. The BILAG Index was a complex paper based assessment and ADS began pioneering the development of a Windows-based database system for lupus assessments, (BILAG, SLEDAI, SLICC, LupusQOL, etc) In 1997 BLIPS was released to the BILAG Group. ADS-Limathon were awarded exclusive licensing rights for the BILAG Index in electronic format and after another two years of validation, BLIPS version 3 was made available to licence from ADS-Limathon. The BILAG group applied their lupus intention to treat principle to the disease myositis, and asked ADS-Limathon developed the MITAX software in 1999. Again MITAX (aka MDAAT) is available in electronic format from ADS-Limaton, and isincorporated in the BLIPS application. BLIPS has evolved through four decades to become the world's most utilised Lupus and Myositis Assessment software.
ADS-Limathon has the unique privelege of being the only BILAG-approved and BILAG-validated computer scoring algorithm to facilitate accurately scoring the BILAG Index. The BILAG Index was relatively unknown in 1996, but is now commonly acknowledged by academics, clinicians and drug licencing authorities worldwide, as the most sensitive lupus assessment index. In BLIPS software, today ADS-Limathon has the market leading product to monitor and assess lupus (SLE), myositis, arthritis, and Sjogrens- all using the intention to treat principle, first pioneered by BILAG in the 1980's and 1990s. patients. The same BLIPS software is used in routine healthcare in clinics or hospitals, in reseach studies, or in pharmacuetical trials of new drugs. In collaboration with the world-class clinical research by the British Isles Lupus Assessment Group BILAG), and software development ADS-Limathon have for over twenty-five years used leading-edge technologies to create, and commercialize, a unique software service to improve lupus patient care, advance lupus research and trial new lupus therapies for patients.
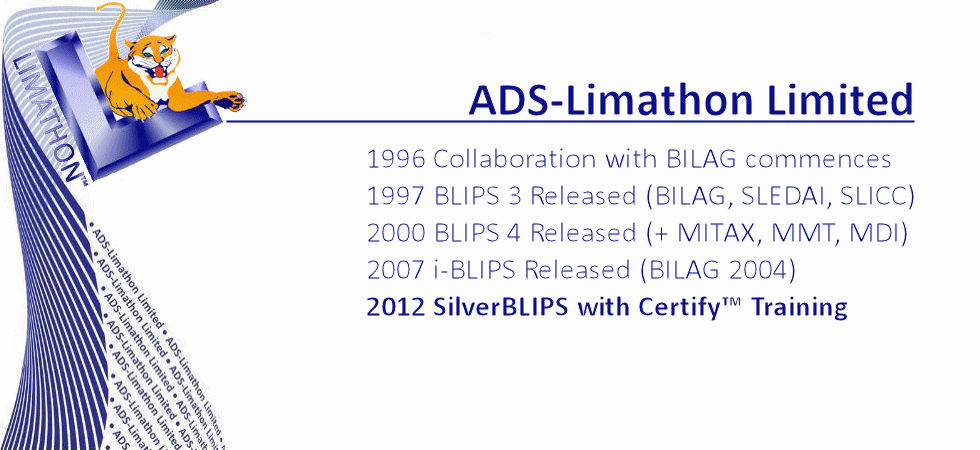
Now in our fourth decade of collaboration with BILAG, ADS-Limathon has also worked closely with several national and international Lupus Assessment Groups, Clinical Research Organisations, and Pharmaceutical Companies to improve the understanding and assessment of SLE. Over the years, BLIPS has iteratively evolved from desktop-installed sofrware into software-as-a-service, now requiring no installation whatsoever. ADS-Limathon provide a complete hosted package of software, database, and hardware. Add to this training and support for clinicians, helpdesk for investigators and study managers and expert data monitoring for studies and today we have the complete solution, scalable come from an individual clinic up to the largest of clinical trials. Our academic sites still hold research data acquired in the 1980s.
Today our research is focused on developing and expanding our BLIPS software as a service, making it easier for clinics to access more assessments, using the latest software and technology. We are constantly increasing the software functionality to improve the quality of data, Through collaboration with our pharma clients and the assistance of the BILAG authors we have pioneered clinical standardisation through online training and exams, automated data checks, on-line adjudication, real-time expert data monitoring, data quality heat-maps, and real-time responder calculation.
The pace of drug discovery and combinational therapies in lupus management is accelerating rapidly, bringing the promise of incredible advances to patients. We are further applying our expertise to make it possible to for patients to be involved in their assessments, by adding their own quality of life and lifestyle data (diet, excercise, wellbeing, etc). This has a many benefits - giving patients more involvement and responsibility towards their wellbeing, whist giving physicians and researchers a more holistic view of contributing disease flare factors, and conversely success factors of disease management. .
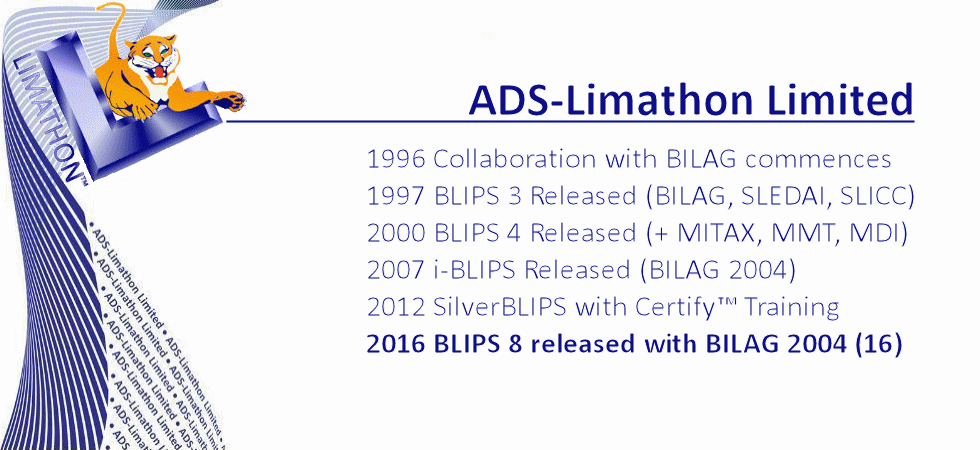
Our aim is to stay at the front of IT technology, and use technology to help patients, physicians and pharma companies combat SLE and other related diseases. We strive to help improve patients' disease management through optimisation of monitoring, recording and analysing of data. In 2014 we made BLIPS available free to UK clinics who helped develop the BILAG index. In 2016 we created BLIPS Global, a worldwide database giving the BLIPS service free to non-profit clinics worldwide. This is helping clinics in countries of all five continents accurately monitor and assess patients, and helping physicians to evaluate and record the patients response to different therapies. It also ensures there is a global standard for BILAG training, still the most sensitive index but the also themost complex in lupus today. Online BLIPS training is also provided. Standardisation is a key factor in ensuring reliablility of data in clinical trials of new lupus drugs. Our BLIPS Global project supports our main object which is to help pharma companies increase their chances of succesful trials, through improved learning, improved investigator standardisation, and higher quality data trial their new drugs to combat SLE. Using the latest technologies, and working closely with CRO's we are able to provide a proven and reliable method of data collection from sites worldwide, score it in real time and provide results instantly to the sites and the study management team.
We always remember, if it's worth doing, it's worth doing well !




 1
1
 2
2
 3
3
 4
4
 6
6
 7
7
 8
8
 9
9
 10
10